
Home |
Our Mission |
Current Stakeholders |
What are Fuel Cells |
News |
Links |
Contact us |

What are fuel cells?
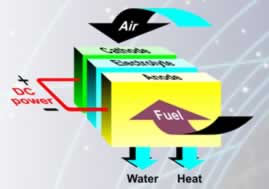
A fuel cell is an electrochemical device that extracts electricity directly
from a fuel (typically hydrogen). A single cell consists of an electrolyte
sandwiched between an anode and a cathode. Hydrogen is supplied to the anode
and a catalyst separates the gas into negatively charged electrons (e-) and
positively charged ions (H+). The electrons (e-) flow through an external
load to the cathode. The hydrogen ions (H+) migrate through the electrolyte
to the cathode where they combine with oxygen and the electrons (e-) to produce
water. Each cell only produces a small voltage (typically 0.7V) and so many
of these are stacked together to give the required level of power.
In contrast to a normal battery, a fuel cell does not become “flat” since
new fuel is continuously supplied to the cell. It can therefore supply constant
power like an engine or a turbine although it does not have any moving parts.
Fuel cells are inherently clean as the only emissions are electricity,
water and heat (when fueled with pure hydrogen). They also offer significant
improvements in efficiency over other energy generation technologies and with
minimal noise (the cell is silent but peripheral equipment for pumping fuel
etc produce some sound).
There are different types of fuel cells as shown in the table below. Currently
the most popular ones are the PEM (for portable, automotive and stand-by power
applications) and SOFC (for larger stationary power generation requirements).
For more information see: www.etscience.co.za
Types of fuel cells
Type |
Electrolyte |
Fuel/oxidant |
Operating temperature (°C) |
Efficiency (%) |
Potential applications |
| Alkaline (AFC) |
Potassium or sodium hydroxide |
H2/O2 (CO2 removed by scrubber) |
50-200 |
40-60 |
Up to 100 kW – space, transport, military |
| Proton exchange membrane (PEM) |
Sulphonic acid in solid polymer membrane |
H2 and O2 from air |
50-125 |
35-45 |
Up to 500 kW – commercial and residential distributed power, transport |
| Direct methanol (DMFC) |
Sulphonic acid in solid polymer membrane or sulphuric acid solution | Methanol and O2 from air |
50-110 |
40-50 |
Up to 10 kW – small, portable power, military, transport |
| Phosphoric acid (PAFC) |
Phosphoric acid | H2 and O2 from air |
170-210 |
40-50 |
Up to 10 MW – power generation, cogeneration (up to 80% efficient), buses |
| Molten carbonate (MCFC) |
Molten lithium, sodium or potassium carbonate | H2 from hydrocarbon fuel internal reforming and O2 from air |
600-700 |
50-60 |
Up to 100 MW – power generation, cogeneration (up to 80% efficient) |
| Solid oxide (SOFC) |
Solid ceramic – zirconium oxide | H2 from hydrocarbon fuel internal reforming and O2 from air |
650-1000 |
45-55 |
Up to 100 MW – power generation, cogeneration (up to 80% efficient), small APU’s for transport |